Here is my list of ionic compounds I found in everyday items. Enjoy...
1. magnesium oxide
MgO
Clif Bar
2. potassium iodide
KI
Clif Bar
3. chromium chloride
chromium (II) chloride: CrCl2
chromium (III) chloride: CrCl3
Clif Bar
4. sodium hypochlorite
NaClO
Clorox Clean-Up with bleach
5. manganese sulfate
manganese (II) sulfate: MnSO4
manganese (III) sulfate: Mn2(SO4)3
Glucosamine Medicine, Triple Strength
6. sodium chloride
NaCl
Opti-free Replenish Contact Solution
7. sodium hydroxide
NaOH
Clean & Clear: Morning Burst Facial Cleanser
8. potassium carbonate
K2CO3
Maruchan Instant Lunch Ramen Noodles (Chicken Flavor)
9. sodium carbonate
Na2CO3
Maruchan Instant Lunch Ramen Noodles (Chicken Flavor)
10. sodium phosphate
Na3PO4
Maruchan Instant Lunch Ramen Noodles (Chicken Flavor)
11. calcium carbonate
CaCO3
Swiss Miss Hot Cocoa Mix
12. calcium sulfate
CaSO4
Friendly Farms-Light Sour Cream
13. calcium chloride
CaCl2
Vlasic Kosher Dill Pickles
14. potassium chloride
KCl
Campbell's Tomato Soup
15. zinc oxide
ZnO
Mary Kay-Timewise Moisturizer
16. sodium fluoride
NaF
Crest Toothpaste
17. ammonium chloride
NH4Cl
Caress-Body Wash
18. magnesium phosphate
Mg3(PO4)2
Flintstones Complete Multimineral Supplement Vitamins
19. phosphoric acid
H3PO4
Diet Coke
20. sodium bicarbonate
NaHCO3
Arm & Hammer-Baking Soda
Wednesday, December 8, 2010
Tuesday, November 9, 2010
Exam Review: 21 c-d
c) Convert 22Mg to 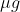
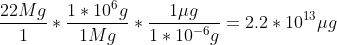
d) If a stack of ten 3.5 inch diskettes is measured to 34 millimeters high, and each disk has a mass of approximately 2.34 grams, how many kilograms would a stack of disks 12.34m high be?
10 diskettes=34mm=0.34cm=23.4g
1 diskette=2.34g
new height=12.34m=1234cm
(Answer: how many diskettes will fit x 2.34g)
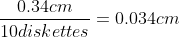 (disk height)
(disk height)
# of disks in new height=1234cm/0.034cm=36,000 (w/ sig figs)
# of disks x 2.34g=weight of new height=84,240g=84.24kg
d) If a stack of ten 3.5 inch diskettes is measured to 34 millimeters high, and each disk has a mass of approximately 2.34 grams, how many kilograms would a stack of disks 12.34m high be?
10 diskettes=34mm=0.34cm=23.4g
1 diskette=2.34g
new height=12.34m=1234cm
(Answer: how many diskettes will fit x 2.34g)
# of disks in new height=1234cm/0.034cm=36,000 (w/ sig figs)
# of disks x 2.34g=weight of new height=84,240g=84.24kg
Tuesday, October 5, 2010
Discovery of the Proton
In deciphering the composition of the atomic nucleus came many experiments. In order to do so, alpha-scattering experiments were popular among scientists. In the early 20th century, Ernest Marsden was curious as to what would happen if alpha rays came in contact with light nuclei. However, Marsden moved to New Zealand for work purposes in 1914, around the beginning of World War I. He was forced to leave his experiments unfinished. Ernest Rutherford decided to take over the experiment from him. However, throughout the war, Rutherford, as well as many other scientists, were preoccupied with figuring out how to detect submarines. From 1914 to 1918, Rutherford left his experiment with the alpha particles to join the war effort. As soon as he was able to, he picked up where he left off before World War I. Rutherford shot alpha particles into nitrogen gas and noticed peculiar reactions.
For his experiment, Rutherford had a brass box and placed a little glass tube inside at one end of the box. A zinc-sulphide scintillation screen was then placed by the glass tube. Then, radon gave off helium nuclei through the glass tube while the brass box was filled with nitrogen. There were then scintillations, or flashes of light, on the screen. Although there was no hydrogen at the beginning of the experiment, the flashes were definitely derived from hydrogen. Rutherford concluded that the nitrogen had disintegrated. Also, in conclusion, Rutherford was convinced from the evidence of his well-thought-out experiment that the hydrogen nucleus was an elementary particle due to the fact that the nitrogen nucleus was made of hydrogen nuclei. Ernest Rutherford named the particle the proton, deriving the word from the Greek word “protos”, which means “first”.
Because Rutherford discovered the proton through a series of steps in his experiment, scientists today are able to determine which chemical element an atom is. The number of protons in the nucleus is equal to the atom’s atomic number, which would not be relevant today without Rutherford’s historical discovery.
Good video (but he means nitrogen instead of neon): http://www.youtube.com/watch?v=iqZpdrEAhTg
Rutherford’s experiment set-up:
Works Cited:
Monday, September 13, 2010
The Physical and Chemical Properties of an Eraser
I chose an eraser as my object to test on because it seemed like an object with not that many chemical properties, but I was determined to find at least one that would change it’s chemical make-up. The process that ended up doing so was the burning of the eraser. The eraser formed into black, or carbon, changing the chemical make-up of the Magic Rub Eraser. Out of the four other chemical properties I tested, none of the rest worked. I feel like a chose a challenging object that would not necessarily react to everything.
Phy1: The eraser’s size is 5.7 cm by 2.4 cm by 0.9 cm. Measuring the eraser using a ruler with a centimeter scale on it can prove this.
Phy2: Color- From observation, the eraser is an eggshell white color.
Phy3: Smell- From observation, the eraser is made of synthetic rubber but it smells like new plastic.
Phy4: Malleability- The eraser is not really malleable. It can bend one way at a 90∘ angle at most.
Phy5: Hardness- The eraser is soft but a solid, so the molecules are close together.
Chem1: Bleach- Nothing happened when the eraser and Clorox bleach came in contact with each other. There was not chemical reaction.
Chem2: Vinegar- When placed in vinegar, the eraser did not react chemically with the liquid. The vinegar just made the eraser the smallest bit more malleable.
Chem3: Fire- When I set the eraser on fire, it gave off a really strong scent of burning rubber. It smelled like there was a car race and the tires were all skidding. The eraser also changed to a dark brown color and shrunk. The eraser reacted chemically with the fire.
Chem4: Boil- When I boiled the eraser for a few minutes, it came out more malleable. It also gave off heat and a smell that smelled like burnt rubber, or car tires that had just skidded. There was no chemical reaction though.
Chem5: Rubbing alcohol- When I put the eraser in rubbing alcohol it became more malleable, but there was no chemical reaction.
Subscribe to:
Posts (Atom)






